Lyme Disease Vaccine Enters Phase 3 Trials
A new vaccine against Lyme disease developed by Pfizer and French drugmaker Valeneva has entered phase 3 trials. The randomized controlled study has enrolled 6,000 volunteers ages 5 and over. The vaccine, VLA15, provoked promising immune responses in adults and children during phase 2 trials. If the phase 3 trials continue to prove safety and efficacy, they hope to apply for authorization in the US and EU by 2025.
“With increasing global rates of Lyme disease, providing a new option for people to help protect themselves from the disease is more important than ever,” said Annaliesa Anderson, Ph.D., Senior Vice President and Head of Vaccine Research & Development at Pfizer.
“We are extremely pleased to reach this important milestone in the development of VLA15. Lyme disease continues to spread, representing a high unmet medical need that impacts the lives of many in the Northern Hemisphere. We look forward to further investigating the VLA15 candidate in Phase 3, which will take us a step closer to potentially bringing this vaccine to both adults and children who would benefit from it,” said Juan Carlos Jaramillo M.D., Chief Medical Officer of Valneva.
How The Vaccine Works
This vaccine is a “multivalent protein subunit vaccine.” Protein subunit vaccines work by containing purified pieces of the pathogen “which have been specially selected for their ability to stimulate immune cells.” The vaccine original series contains three doses, followed by a booster shot.
The VLA15 vaccine targets a specific protein (called OspA) on the surface of the Borrelia burgdorferi bacteria, which causes Lyme disease. After harmless forms of this protein are injected, the body produces an immune response that can prevent infection if bitten by an infected tick. This vaccine improves on previous Lyme disease vaccines by leaving out a protein region that may cause adverse effects, according to the NIAID as reported by NPR.
Examples of other vaccines that use this technology are the hepatitis B vaccine, the whooping cough vaccine, and the Novavax COVID-19 vaccine.
Lyme Disease
Lyme Disease is caused by Borrelia burgdorferi bacteria transmitted through the bite of an infected blacklegged tick. In the US, infected ticks are clustered in the northeast and north-central United States. In Europe, countries in central Europe report the highest rates of infection, including the Czech Republic, Estonia, Lithuania, and Slovenia. Ticks need to be attached for over 24 hours in order to transmit infection.

Map from CDC.gov. This map only shows cases that were actively reported to the CDC, which is only a fraction of total treated cases (ie there is probably more Lyme disease in MA than this map implies).
According to the CDC, there are almost 500,000 cases of Lyme disease diagnosed and treated every year (though this number may be an overestimate). Symptoms include:
- Fever/Chills
- Headache
- Fatigue
- Body aches
- Swollen lymph nodes
These are accompanied by an Erythema Migrans rash, which looks like a target sign around the bite.
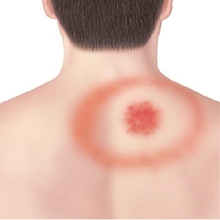
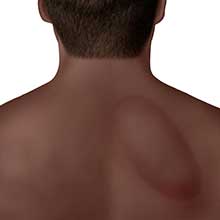
Illustrations from the CDC
As the disease progresses, it may cause:
- Severe headaches/neck stiffness
- Arthritis
- Facial Palsy (drooping or loss of muscle tone in one or both sides of the face)
- Joint, tendon, muscle, and bone pain
- Heart palpitations/irregular heartbeat
- Dizziness/shortness of breath
- Brain/spinal cord inflammation
- Nerve pain
- Pain, numbness, or tingling in the hands or feet
Lyme disease is treatable with antibiotics and is usually not fatal. In some cases, symptoms can persist after treatment or can cause permanent damage. The earlier diagnosis and treatment occur, the less likely the disease is to progress to more severe symptoms.
History of Lyme Vaccine Attempts
If this vaccine is approved, it will be the first Lyme vaccine in 20 years, since LYMErix was taken off the market in 2002 due to declining sales. According to an article in the National Library of Medicine, the vaccine was initially received positively and marketed widely in 1998, before a high-profile lawsuit in 1999 that accused the manufacturer of minimizing the risk and severity of vaccine side effects. In particular, they claimed that the vaccine caused arthritis in some subjects with certain genetic predispositions.
The FDA “found no suggestion that the Lyme vaccine caused harm to its recipients,” but poor press coverage and lingering safety concerns led to plummeting sales. This vaccine also coincided with growing vaccine skepticism in the US, especially following the revocation of the rotavirus vaccine for infants in 1999.
Featured image: (Left to right) blacklegged tick larva, nymph, adult female, adult male. Blacklegged ticks can transmit Lyme disease via their bite. Photo via Fairfax County.
This website contains affiliate links, which means The Trek may receive a percentage of any product or service you purchase using the links in the articles or advertisements. The buyer pays the same price as they would otherwise, and your purchase helps to support The Trek's ongoing goal to serve you quality backpacking advice and information. Thanks for your support!
To learn more, please visit the About This Site page.

 ">
">

